RISK ANALYSIS – ISO 14971
It is perhaps the most important section of the Technical File: the RISK ANALYSIS! The standard which ha set the used structure
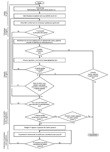
in the harmonized standards of the different EU directives/(UE) regulations, it is EN 14971, the norm used to manage the medical devices’ risks especially during the development cycle from design to disposal.
The Manufacturer knows that the analysis must be carried out by aninterdisciplinary team at least composed by:
- an expert of the product (generally the designer),
- a tipical user (generally the opinion leader),
- and an expert of the risk management techniques.
The first two figures, almost always reside within the company, while for the third, it is established that knowledge hardly resides in the organization. The ‘copying and pasting’ of the risk analysis of the previous product is itself too risky a modus operandi, because it does not focus on specific risks but on common risks, which do not always correspond to the disadvantage of identifying more effective and efficient measures to reduce risks, the severity and probability index, as reasonably as possible.
We EVALUATORS of Notified Bodies, we always pay much and more and more attention to this important analysis, as anticipated, an integral part of the Technical Dossier. Why not do it right? Why not just focus on sales and thus generate profits, but worry about the onset of an unethused adverse event in risk analysis?
MEQUIPE offers its experience gained in over 30 years of activity in the field of product and system certification, especially in the medical field, to support you in the drafting of the most important analysis document of the technical dossier: RISK ANALYSIS!
Ask for a Quote
Scarica il Modulo per la richiesta di un preventivo




